RadLex Playbook Series
DICOM imaging studies frequently include multiple image series, each acquired using a distinct protocol (e.g., view, phase, contrast). These series are described in widely varying ways by different device manufacturers and sites, which raises a number of issues in the implementation of hanging protocols, clinical and business operations in radiology and aggregation and analysis of imaging data.
Building on the success of the Playbook project, the RadLex Committee has initiated work to address these issues and have assembled a task force of radiologists and informaticists to develop a set of standardized, vendor neutral, imaging-series names.
The committee includes members from academic and private practice as well as industry.
Task force members
Ross W. Filice, MD; MedStar Georgetown, Radlex Chair
Audrey Verde, MD, PhD, Duke University, Radlex Vice Chair
Beverly Collins; Penn Medicine
R. Kent Hutson, MD; Rad Partners
Marc D. Kohli, MD; UCSF
John Mongan, MD, PhD; UCSF
Gloria L. Hwang, MD; Stanford University
Charles E. Khan, MD, MS; Penn Medicine
Kenneth C. Wang, MD, PhD; University of Maryland School of Medicine
Nina Kottler MD, MS; Rad Partners
George L. Shih, MD, MS; Weill Cornell Medical
Timothy P. Szczykutowicz, PhD; University of Wisconsin, Madison
Jason M. Hostetter, MD; Wellspan Health
Stacy O’Connor, MD; UNC
Jacob Bernhard, Sectra
Beth Santori; Enlitic
Greg Zaharchuk, MD, PhD; Subtle Medical
RadLex Playbook Standard Series Names
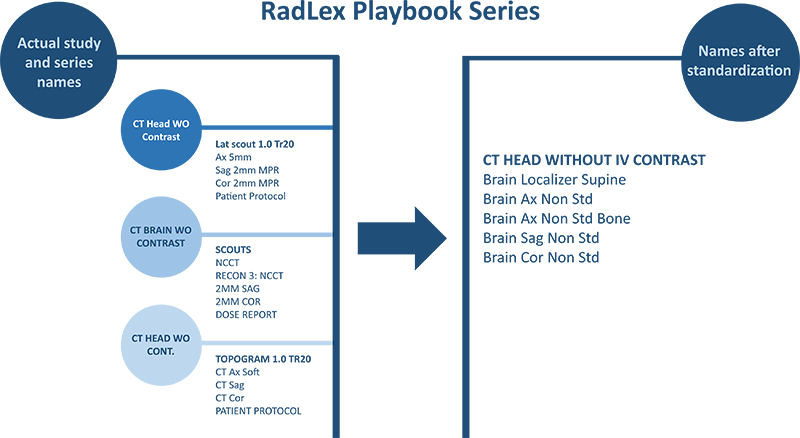
Proposed CT series naming convention
The Study Series Standardization Task Force created an interactive tool for the public to test and provide feedback for the proposed CT convention. Enter your institution’s series data and watch the suggested series name appear.
Test the RadLex Playbook Series
Laterality (optional)
Body Part
Anatomic Plane
IV Contrast
Luminal Contrast (optional)
Series Type
Spectral Energy (optional)
Slice Thickness (required)
Kernel (optional)
Positioning (required)
Projection Imaging
Repeat #
Share your feedback with us
After you test the RadLex Playbook Series, be sure to share your thoughts with us. Your feedback is valuable and will guide product improvements.
Convention philosophy
- Vendor neutral
- Applicable across practice settings
- Applicable across subspecialties
- Provides clinically useful information that would inform the radiologist which series to select
- Includes body part up front, to assist with identifying series for multi-body part acquisitions
- Data that is not necessary for series selection and that can be found within the DICOM is not necessary to include
- To focus on the most common and clinically useful series name elements—with the understanding that it is not possible to be all inclusive while remaining non-prescriptive of exam protocols
Convention goals
To provide a standardized format for the most common series-name elements to achieve consistency of data across scanners and institutions allowing for hanging protocols to function reliably.
Secondary gain of series naming convention
- Standard series data elements will facilitate research efforts
- Standard series data elements will facilitate algorithm development, training, testing and utilization
Initial convention scope
The proposed series naming convention was developed with CT in mind, as this modality is widely available, allowing for diverse application, testing and feedback.
The next phase of the series standardization project is to collaborate with our industry partners to expand the naming convention for MRI series application.
Contact us
For questions, please email informatics@rsna.org.
FAQs
How is slice thickness defined?
- Recon: < 1 mm
- Thin: >= 1 mm and < 2.5 mm
- Std: >= 2.5 mm and < 5 mm
- Thick: >= 5 mm
How long can a series name be?
Are there additional convention rules?
- All series descriptions will use Title Case
- Space is the only allowed separator between series description elements; underscore is the only allowed separator within elements