MRI Tips for Imaging Adults With Implanted Devices
Radiologists should be involved in patient screening and monitoring patients before, during and after MRI


As active implanted medical devices (AIMDs) become increasingly more commonplace in the MRI suite, radiologists should be ready to modify acquisition protocols, weigh potential benefits and risks, and consider alternative modalities to aid diagnosis while keeping their patients safe.
Some of the most common types of AIMDs are cardiac implantable electronic devices; deep brain, spinal cord and other neurostimulators; bone growth or fusion stimulators; and drug infusion pumps, said Matthew Harwood, MD, an assistant professor at Creighton University and chief of nuclear medicine at St. Joseph’s Hospital and Medical Center in Phoenix.
In a RadioGraphics article, Dr. Harwood and colleagues give practical advice on calculating MRI protocols based on manufacturer specifications and guidance on determining safety factors when a device hasn’t been tested in an MRI environment.
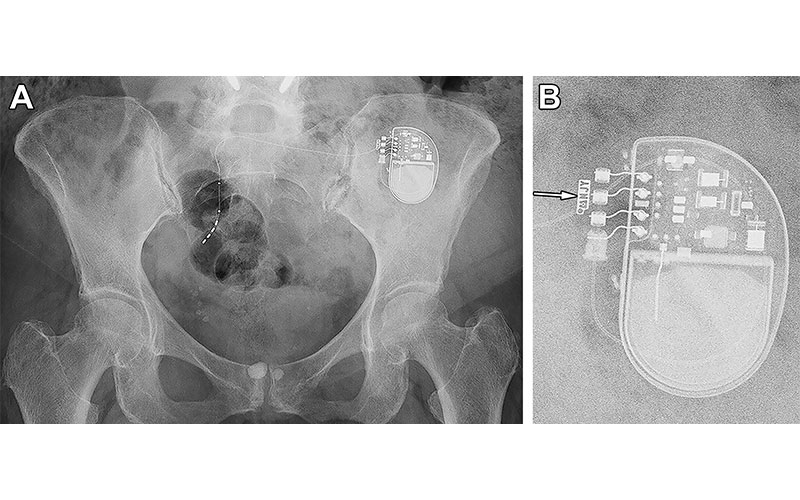
Radiographic identification of a sacral neurostimulator device in a 77-year-old woman. (A) Frontal projection radiograph of the pelvis shows a sacral neurostimulator device. (B) Magnified radiographic view of the device reveals the manufacturer symbol and identification code NJY (arrow), indicating that this device is a model 3058 InterStim II neurostimulator (Medtronic). https://doi.org/10.1148/rg.230102 © RSNA 2024
Those untested devices, which the authors term “MR non-conditional,” should be considered on a case-by-case-basis. And Dr. Harwood and his co-authors also recommended personalized consideration with a device of any type, MR-tested or not. In the article, they outlined clinical considerations and suggested approaches to address:
- Translational force and torque. The static magnetic field around an MRI system varies in magnitude with respect to distance, creating a spatial gradient magnetic field. This shouldn’t be confused with the time-varying gradient magnetic fields produced by the gradient coils, the authors cautioned. The peak spatial gradient occurs in the physical space around the magnet, usually at the cover of a closed-bore unit. Medical devices are at highest risk for device migration near this peak. Meanwhile, torque is greatest at the magnet’s isocenter and increases as the angle of the implant moves away from the static line of the magnetic field.
- Induced current that results in tissue or device stimulation. Device manufacturers specify a maximal slew rate or the measure of how quickly the time-varying and spatially varying gradient, the gradient for spatial localization, reaches its maximal strength. This rate can help keep induced currents to a minimum, Dr. Harwood said.
Manufacturers also may specify the simpler maximal dB/dt or change in magnetic field per unit time. These parameters affect spatial resolution, signal-to-noise ratio, imaging time, distortion and metal susceptibility artifacts.
“MRI systems enable the selection of high, medium and low peak gradients, effectively placing an upper limit on the slew rate and, therefore, on the rate of change of the gradient magnetic field,” Dr. Harwood said.
Sequences with rapidly switching gradients—such as echo-planar and single-shot sequences—pose the highest risk.
- Heating. Often the most restrictive parameter in MRI, heating is frequently addressed by sacrificing imaging time, signal-to-noise ratio, or spatial resolution. Therefore, radiologists look to the parameter modifications that yield the most reduction in specific absorption rate while leaving other parameters relatively unchanged. Resonant heating of devices is another safety hazard, the authors noted.
“The practical consideration is that manufacturers set safety limits in SAR or B1+RMS for every device, and the operator has to meet or beat (stay under) the heating requirements. There’s also an actual limit for gradient output, which again the operator has to stay under. Usually the gradient output isn’t a big limiting factor,” Dr. Harwood said. “Choose any modern AIMD and it will list the safety requirements in its manual.”
Radiologists Are Critical to Determining Necessity of MRI
While MRI was once restricted to specialized academic centers with specially trained staff, access to MRI will—and should—continue to grow to accommodate patients who need access to high-quality imaging, said Anup Shetty, MD, who coauthored an invited commentary in RadioGraphics. He emphasized the importance of radiologists’ involvement before a patient enters the MRI suite.Dr. Shetty, an associate professor at Washington University’s Mallinckrodt Institute of Radiology in St. Louis, stressed that radiologists must play an active role in the initial evaluation and approval of MRI examinations in patients with AIMDs.
“Many MRI studies are ordered reflexively as a consequence of recommendations from another imaging study,” Dr. Shetty said. “Others may be requested without a specific clinical question or owing to the desire of the patient. The radiologist is best suited to understand the need for MRI and recommend alternative diagnostic examinations that can address the clinical matter at hand.”
Challenges with staffing of MR technologists place a premium on experienced technologists, particularly those with MR safety officer (MRSO) expertise, Dr. Shetty added. Radiologists should work in concert with the facilities that perform MRI exams to help ensure that their staff have sufficient expertise to safely scan patients with AIMDs.
For More Information
Access the RadioGraphics paper, “MRI in Adult Patients with Active and Inactive Implanted MR-conditional, MR-nonconditional, and Other Devices,” and the accompanying commentary, “MRI in Patients with Active Implanted Medical Devices: Demand Will Only Grow.”
Read previous RSNA News stories about MRI: