Developing Imaging Metrics to Personalize Brain Cancer Treatment
R&E Foundation grant uncovers associations between imaging features and pathological diagnoses


In 2016, and more recently in 2021, the World Health Organization (WHO) updated the classification of adult diffuse gliomas based on molecular markers. The brain cancers are now defined by the presence or absence of a mutation in the gene that encodes isocitrate dehydrogenase (IDH), an enzyme that drives the earliest events in oncogenesis. The tumors are also defined now by their 1p/19q chromosome status, a genetic marker predictive of therapeutic response to treatment.
However, to determine the genetic subtype, grade and diagnosis of brain tumors, surgical pathology is still required.
An R&E Foundation grant research project by Sohil H. Patel, MD, uncovered associations between the CT and MRI features of gliomas and their pathological diagnoses. His 2018 RSNA Research Scholar Grant entitled, “Radiogenomics of Diffuse Cerebral Gliomas,” sheds light on the ability of imaging metrics to inform the treatment plans of brain cancer patients.
“Prognostic and therapeutic biomarkers in diffuse glioma are becoming increasingly well understood in recent years,” explained Dr. Patel, associate professor of radiology and medical director of MRI at the University of Virginia (UVA). “These biomarkers have the potential to assist in the development of effective and personalized patient care.”
The Future of Imaging Gliomas
Dr. Patel sought to characterize the neuroimaging phenotypes of the WHO glioma subtypes through both an independent human reader and a deep learning neural network analysis of CT and MRI features. His secondary research goal was to examine whether neuroimaging could inform clinically meaningful classification of diffuse gliomas beyond the WHO paradigm. Moreover, gliomas are inherently heterogeneous in composition and therefore can be subject to sampling error. Image-based mapping may offer a better means to “survey” the entire tumor.
“There’s a lot of unique information provided by imaging that isn’t necessarily captured in the current classification system for gliomas. The purpose of my research is to more usefully apply imaging metrics on a case-by-case basis to help inform the individual patient’s treatment plan.”
Sohil H. Patel, MD
“The ultimate long-term goal would be to diagnose gliomas non-invasively via imaging but we’re not there yet, in part because surgical resection is performed for both diagnostic and therapeutic purposes,” Dr. Patel said.
However, for gliomas that cannot be treated surgically, Dr. Patel posits that diagnosis through imaging offers great potential utility.
“There’s a lot of unique information provided by imaging that isn’t necessarily captured in the current classification system for gliomas,” Dr. Patel said. “The purpose of my research is to more usefully apply imaging metrics on a case-by-case basis to help inform the individual patient’s treatment plan.”
MRI Metrics Can Help Improve Diagnosis
Dr. Patel’s research also investigated the ability of neuroimaging to uncover cases of misclassification by fluorescence in situ hybridization (FISH), the most commonly performed pathology assay for 1p/19q determination. According to Dr. Patel, FISH has a 5-10% error rate.
In the study, Dr. Patel’s team analyzed neuroimaging features of 112 IDH-mutant low-grade gliomas (LGGs) where 1p/19q co-deletion status was determined by FISH.
In nine of the LGGs, the neuroradiology prediction of co-deletion status, or molecular alteration, differed from the FISH results. Those cases were re-tested with a different molecular assay and in six of the nine cases, the FISH result was inaccurate.
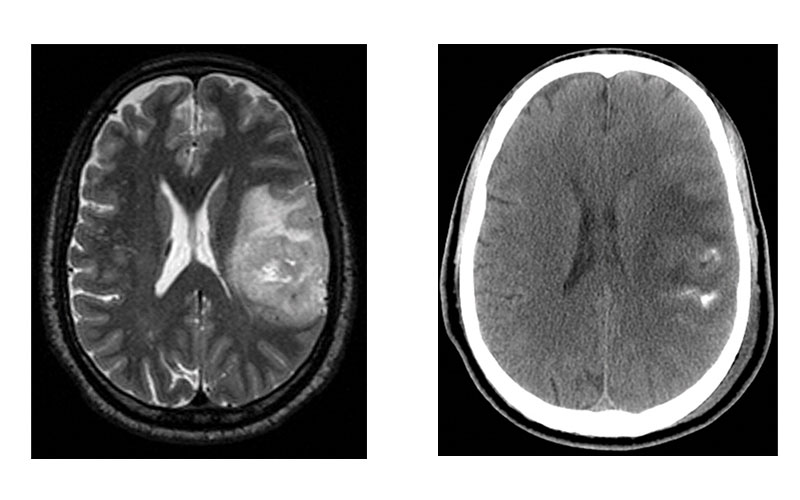
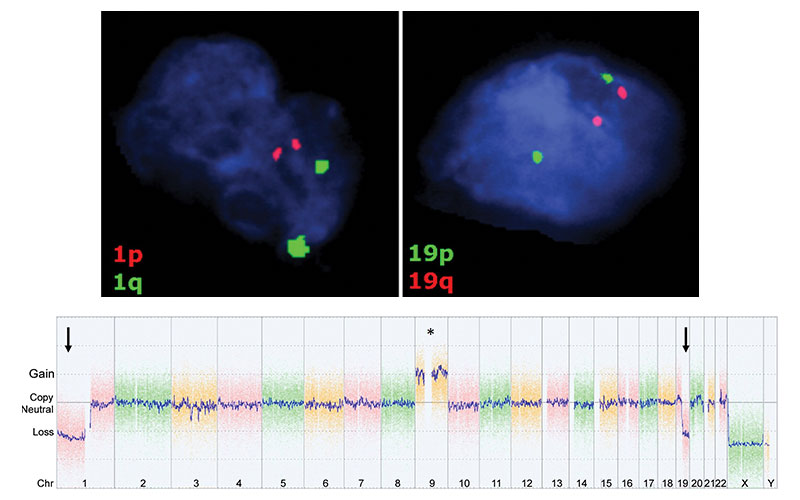
Images in representative case of disagreement between neuroradiologist and fluorescence in situ hybridization (FISH) classification in a 53-year-old man with an isocitrate dehydrogenase–mutant lower-grade glioma. (a) Axial image from T2-weighted MRI shows a left frontoparietal mass with marked internal heterogeneity and extensive cortical infiltration. (b) Axial noncontrast CT scan shows calcifications associated with the mass. On the basis of the imaging findings, the consensus neuroradiologist prediction for 1p/19q was “codeleted.” (c) FISH showed no evidence of 1p/19q codeletion. (d) Chromosomal (Chr) microarray analysis showed 1p/19q codeletion (arrows), as well as trisomy for chromosome 9 (*).
Sohil et al Radiology 2020; 294:160–167 ©RSNA 2020
The results, according to Dr. Patel, establish a novel and clinically significant role for neuro-imaging in determining an accurate classification for IDH-mutant LGGs.
“As radiologists, we view a pathology diagnosis as the gold standard and the final say on diagnoses,” Dr. Patel said. “But as good as pathology is at classifying gliomas, it isn’t 100% accurate. MRI metrics can improve that accuracy.”
While imaging features aren’t making the ultimate diagnoses, Dr. Patel said they are especially useful in cases with small or incomplete pathologic samples, as well as for supplementing pathologic classifications that are known to be imperfect, such as tumor grade assignment.
“We are developing a good sense of the imaging features that associate with specific tumor grades and providing correlations on an individual basis that can help establish the most accurate diagnosis,” he said.
Dr. Patel hopes to continue working on radiogenomics in the future, including identifying specific non-invasive biomarkers to surveil tumors.
“Right now, there are no non-invasive biomarkers that definitively tell physicians if tumors have progressed or recurred that don’t overlap with other conditions,” Dr. Patel said. “Those biomarkers would be extraordinary to have.”
R&E Foundation Grant Offers Support
Dr. Patel said the RSNA Research Scholar Grant afforded him the opportunity to spend time strengthening relationships and making new connections with colleagues from varied disciplines, including neuro-oncologists, computer scientists, and biomedical engineers.
His experience in busy neuro-oncology practices including UVA and NYU Langone Health exposed him to the cutting edge of the field and connected him with a great mentor in neuro-radiologist Rajan Jain, MD, a neuroradiologist and professor of radiology at NYU, who helped guide his research.
“This work benefited both my collaborators and me personally,” Dr. Patel said. “It showed how productive we can be when we make use of all our skill sets.”
For More Information
Learn more about R&E funding opportunities.
Read previous RSNA News stories on R&E Foundation grant recipients: