Fewer Recalls Associated with Higher Rates of Interval Breast Cancers
Establishing a minimum recall rate is a potentially important goal for breast cancer screening programs

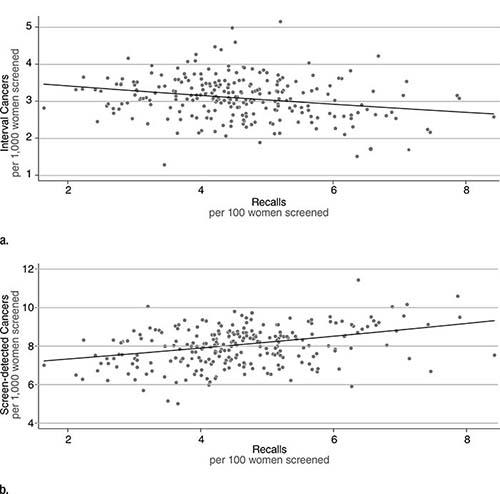
Lower screening mammography recall rates are associated with higher rates of breast cancers detected between screenings, or interval cancers, according to a new study in Radiology.
Researchers analyzed 5,126,689 screening episodes, or the period during which a set of breast screening activities for an eligible woman takes place, from 84 screening facilities in the U.K. National Health Service Breast Screening Program (NHSBSP). Data were drawn from a 36-month period with a three-year follow-up in women ages 50 to 70 years.
Recall for additional testing after mammography is an important safeguard against missing invasive breast cancers, but the practice carries costs, including patient anxiety and additional expenses to the healthcare system. In order to minimize these costs, breast cancer screening programs routinely specify maximum recall rates after screening mammography.
“The U.K. has established a maximum recall rate target of less than 7 percent for a patient’s first screening, also known as a prevalent screen, and less than 5 percent for incident screens, or those in which previous screening results exist,” said co-author Elizabeth S. Burnside, MD, MPH, from the University of Wisconsin School of Medicine and Public Health in Madison, WI. “However, no such consensus exists as to a minimum recall rate, below which additional cancers would be missed.”
Recall rate averaged 4.56 percent, with an interval cancer rate of 3.1 per 1,000 women screened. Interval cancers are associated with a worse prognosis than screen-detected cancers.
Age Can Affect Cancer Detection and Interval Cancer Rates
Lower recall rates correlated with higher interval cancer rates. Dr. Burnside and colleagues estimated that, in aggregate, 80 to 84 additional recalls would be required to avoid one interval cancer, a ratio that varied based on age group and prevalent screens versus incident screens.
Screening mammography outcomes based on age demonstrated that both cancer detection rates and interval cancer rates were lower in younger age groups as expected, based on underlying breast cancer incidence according to age.
For women aged 50-54 years, there was one interval cancer per 89-96 recalls versus the one interval cancer per 55-58 recalls for women aged 65-69 years.
“Recall rate had more of an impact on interval cancer in patients who are older,” Dr. Burnside said. “The lower number of recalls required per interval cancer avoided in older women and incident screens as compared to younger women and prevalent screens, respectively, demonstrates a slightly different ‘value’ in terms of the trade-off between recall rate and interval cancers.”
The study provides evidence, Dr. Burnside said, of the potential importance of establishing and enforcing a lower threshold for recall rate.
While the study focused on the U.K. NHSBSP alone, Dr. Burnside pointed out that the methods used provide a basis for other programs to determine a minimal recall rate threshold that maximizes value for women undergoing breast cancer screening.
The study also underscores the crucial importance of comprehensive, accurate data collection of interval cancers in screening programs like the system in the U.K.
“A big-picture lesson in our study is the power of rigorous quality assurance infrastructure to help breast cancer screening programs learn from actual practice and use that information to make informed programmatic decisions for the future,” Dr. Burnside said. “This study documents the benefit of carefully tracking mammography use and outcomes as achieved in the NHSBSP.”
Additional research will help determine a specific minimum threshold for recall rate and assess the impact of digital mammography on the relationship between recall rate and interval cancers.
Web Extras
- Access the study, “Association between Screening Mammography Recall Rate and Interval Cancers in the UK Breast Cancer Service Screening Program: A Cohort Study,” at https://pubs.rsna.org/doi/full/10.1148/radiol.2018171539